Publications
Highlights
The Architecture of SARS-CoV-2 Transcriptome
SARS-CoV-2 is a betacoronavirus responsible for the COVID-19 pandemic. We have sequenced both genomic and subgenomic RNAs to understand the transcriptional landscape of the viral activities. Two complementary techniques were adopted for this study. DNA nanoball sequencing shows that the transcriptome is highly complex, owing to numerous discontinuous transcription events. SARS-CoV-2 produces transcripts encoding unknown ORFs with fusion, deletion, and frameshift in addition to the canonical genomic and nine subgenomic RNAs. Using nanopore direct RNA sequencing, we mapped the primary structures of full-length subgenomic RNAs with discontinuities. The relatively new technique also enables the analysis of RNA modifications. We found frequent modifications that are specific to a subset of viral RNAs. Functional investigation of the unknown transcripts and RNA modifications discovered in this study will open new directions to our understanding of the life cycle and pathogenicity of SARS-CoV-2.
1,
,
,
,
*, and
Hyeshik Chang*
Cell, 181(4):914-921.e10
Terminal Uridylyltransferases Execute Programmed Clearance of Maternal Transcriptome in Vertebrate Embryos
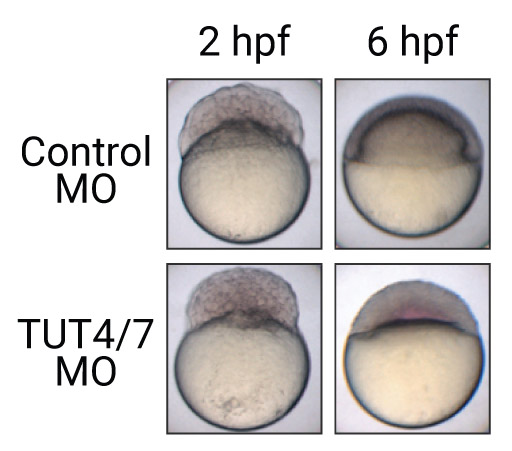
Life starts with the RNAs and proteins derived from its mother in animals. For the first few cell cycles, maternally deposited RNAs are precisely used to produce the right amount of the proteins at the right place. Once the bootstrapping of embryo finishes, the maternal RNAs are replaced by the zygotically transcribed RNAs. In this paper, we surveyed the poly(A) tails during the earliest cell cycles of vertebrate embryos to understand how the transition is accurately controlled. A terminal uridylyltransferase, TUT7, is found to be a driving factor initiating maternal RNA degradation. The preference to short poly(A) tails of TUT7 contributes to the selectivity of the process. This paper demonstrates the unique opportunities of TAIL-seq in the studies of biological phenomena coming from its unprecedented throughput and resolution in contrast to the existing biochemical methods.
Hyeshik Chang1,
1,
,
,
,
,
,
,
,
,
,
, and
*
Molecular Cell, 70(1):72–82
Full List
‣
SARS-CoV-2 infection engenders heterogeneous ribonucleoprotein interactions to impede translation elongation in the lungs
Junsoo Kim1,
1,
1,
,
,
,
Hanju Lee,
,
,
,
,
,
,
,
,
,
,
,
,
,
,
,
,
,
*,
Hyeshik Chang*,
*, and
*
Experimental & Molecular Medicine
(2023), in press
‣
Functional and Molecular Dissection of HCMV Long Non-coding RNAs
1,
1,
Ari Hong1,
1,
,
,
,
,
,
,
,
,
Hyeshik Chang*, and
*
Scientific Reports
(2022), 12:19303
‣
Analyzing Viral Epitranscriptomes Using Nanopore Direct RNA Sequencing
Ari Hong1,
1,
*, and
Hyeshik Chang*
Journal of Microbiology
(2022), 60:867–876
Partitioning RNAs by Length Improves Transcriptome Reconstruction from Short-Read RNA-seq Data
1,
1,
,
,
,
,
Ari Hong,
,
,
,
Hyeshik Chang,
,
,
,
, and
*
Nature Biotechnology
(2022), 40:741–750
Viral Hijacking of the TENT4–ZCCHC14 Complex Protects Viral RNAs via Mixed Tailing
1,
1,
1,
1,
,
,
,
Hyeshik Chang,
,
,
,
,
, and
*
Nature Structural & Molecular Biology
(2020), 27:581–588
‣
The Architecture of SARS-CoV-2 Transcriptome
1,
,
,
,
*, and
Hyeshik Chang*
Cell
(2020), 181(4):914–921.e10
Development of a Laboratory-safe and Low-cost Detection Protocol for SARS-CoV-2 of the Coronavirus Disease 2019 (COVID-19)
,
,
,
,
,
,
,
Hyeshik Chang,
, and
*
Experimental Neurobiology
(2020), 29(2):107–119
Bias-Minimized Quantification of MicroRNA Reveals Widespread Alternative Processing and 3′ End Modification
1,
1,
,
Hyeshik Chang,
, and
*
Nucleic Acids Research
(2019), 47(5):2630–2640
Mixed Tailing by TENT4A and TENT4B Shields mRNA from Rapid Deadenylation
1,
1,
1,
,
,
,
Hyeshik Chang,
,
, and
*
Science
(2018), 361(6403):701–704
PABP Cooperates with the CCR4-NOT Complex to Promote mRNA Deadenylation and Block Precocious Decay
1,
1,
,
,
Hyeshik Chang, and
*
Molecular Cell
(2018), 70(6):1081–1088.e5
‣
Terminal Uridylyltransferases Execute Programmed Clearance of Maternal Transcriptome in Vertebrate Embryos
Hyeshik Chang1,
1,
,
,
,
,
,
,
,
,
,
, and
*
Molecular Cell
(2018), 70(1):72–82
mTAIL-seq Reveals Dynamic Poly(A) Tail Regulation in Oocyte-to-Embryo Development
1,
1,
,
Hyeshik Chang, and
*
Genes & Development
(2016), 30:1671–1682
Regulation of Poly(A) Tail and Translation During the Somatic Cell Cycle
1,
1,
1,
Hyeshik Chang, and
*
Molecular Cell
(2016), 62(3):462-471
Next-Generation Libraries for Robust RNA Interference-Based Genome-Wide Screens
1*,
,
,
,
,
,
,
,
Hyeshik Chang,
, and
*
Proceedings of the National Academy of Sciences of the U. S. A.
(2015), 112(26):E3384-E3391
Temporal Landscape of MicroRNA-Mediated Host-Virus Crosstalk during Productive Human Cytomegalovirus Infection
1,
1,
,
,
Hyeshik Chang,
,
,
, and
*
Cell Host & Microbe
(2015), 17(6):838–851
TUT7 Controls the Fate of Precursor MicroRNAs by Using Three Different Uridylation Mechanisms
1,
1,
1,
Hyeshik Chang,
,
,
,
,
*, and
*
EMBO Journal
(2015), 35(2):115-236
‣
miRseqViewer: Multi-Panel Visualization of Sequence, Structure and Expression for Analysis of MicroRNA Sequencing Data
1,
Hyeshik Chang1,
,
,
,
,
,
, and
*
Bioinformatics
(2015), 31(4):596–598
‣
Uridylation by TUT4 and TUT7 Marks mRNA for Degradation
1,
1,
Hyeshik Chang1,
,
,
, and
*
Cell
(2014), 159(6):1365–1376
‣
TAIL-seq: Genome-wide Determination of Poly(A) Tail Length and 3′ End Modifications
Hyeshik Chang1,
1,
, and
*
Molecular Cell
(2014), 53(6):1044–1052
‣
FitSearch: a Robust Way to Interpret a Yeast Fitness Profile in Terms of Drug’s Mode-of-Action
1,
1,
Hyeshik Chang1,
,
, and
*
BMC Genomics
(2014), 14(Suppl 1):S6
Construction of the First Compendium of Chemical-Genetic Profiles in the Fission Yeast Schizosaccharomyces pombe and Comparative Compendium Approach
1,
1,
Hyeshik Chang,
,
,
,
,
,
,
*, and
*
Biochemical and Biophysical Research Communications
(2013), 436(4):613–618
miRGator v3.0: a MicroRNA Portal for Deep Sequencing, Expression Profiling and mRNA Targeting
1,
,
,
,
,
,
,
Hyeshik Chang,
,
,
,
*, and
*
Nucleic Acids Research
(2013), 41(D1):D252–D257
‣
LIN28A is a Suppressor of ER-associated Translation in Embryonic Stem Cells
1,
1,
,
,
,
,
,
, and
*
Cell
(2012), 151(4):765–777
Mono-Uridylation of Pre-MicroRNA as a Key Step in the Biogenesis of Group II Let-7 MicroRNAs
1,
1,
,
,
,
,
Hyeshik Chang, and
*
Cell
(2012), 151(3):521–532
Dicer recognizes the 5′ end of RNA for efficient and accurate processing
1,
1,
,
,
Hyeshik Chang,
,
, and
*
Nature
(2011), 475(7355):201-205

